Cellulose
 | |
 | |
| Identifiers | |
|---|---|
CAS Number |
|
ChEMBL |
|
ChemSpider |
|
ECHA InfoCard | 100.029.692 |
EC Number | 232-674-9 |
E number | E460 (thickeners, ...) |
PubChem CID |
|
UNII |
|
| Properties | |
Chemical formula | (C 6H 10O 5) n |
Molar mass | 162.1406 g/mol per glucose unit |
| Appearance | white powder |
Density | 1.5 g/cm3 |
Melting point | 260–270 °C; 500–518 °F; 533–543 K Decomposes[2] |
Solubility in water | none |
| Thermochemistry | |
Std enthalpy of formation (ΔfH | −963,000 J/mol |
Std enthalpy of combustion (ΔcH | −2828,000 J/mol |
| Hazards | |
NFPA 704 |  1 1 0 |
| US health exposure limits (NIOSH): | |
PEL (Permissible) | TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
REL (Recommended) | TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
IDLH (Immediate danger) | N.D.[2] |
| Related compounds | |
Related compounds | Starch |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
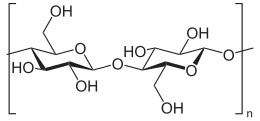
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.[3][4] Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms.[5] Cellulose is the most abundant organic polymer on Earth.[6] The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.[7][8][9]
Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton.[6]
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha. In human nutrition, cellulose is a non-digestible constituent of insoluble dietary fiber, acting as a hydrophilic bulking agent for feces and potentially aiding in defecation.
Contents
1 History
2 Structure and properties
3 Processing
3.1 Assay
3.2 Biosynthesis
3.3 Breakdown (cellulolysis)
3.4 Breakdown (thermolysis)
4 Hemicellulose
5 Derivatives
6 Applications
7 See also
8 References
9 External links
History
Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula.[3][10][11] Cellulose was used to produce the first successful thermoplastic polymer, celluloid, by Hyatt Manufacturing Company in 1870. Production of rayon ("artificial silk") from cellulose began in the 1890s and cellophane was invented in 1912. Hermann Staudinger determined the polymer structure of cellulose in 1920. The compound was first chemically synthesized (without the use of any biologically derived enzymes) in 1992, by Kobayashi and Shoda.[12]

The arrangement of cellulose and other polysaccharides in a plant cell wall.
Structure and properties
Cellulose has no taste, is odorless, is hydrophilic with the contact angle of 20–30 degrees,[13] is insoluble in water and most organic solvents, is chiral and is biodegradable. It was shown to melt at 467 °C in 2016.[14] It can be broken down chemically into its glucose units by treating it with concentrated mineral acids at high temperature.[15]
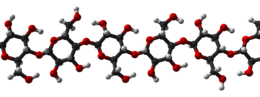
Cellulose is derived from D-glucose units, which condense through β(1→4)-glycosidic bonds. This linkage motif contrasts with that for α(1→4)-glycosidic bonds present in starch and glycogen. Cellulose is a straight chain polymer: unlike starch, no coiling or branching occurs, and the molecule adopts an extended and rather stiff rod-like conformation, aided by the equatorial conformation of the glucose residues. The multiple hydroxyl groups on the glucose from one chain form hydrogen bonds with oxygen atoms on the same or on a neighbor chain, holding the chains firmly together side-by-side and forming microfibrils with high tensile strength. This confers tensile strength in cell walls, where cellulose microfibrils are meshed into a polysaccharide matrix.

A triple strand of cellulose showing the hydrogen bonds (cyan lines) between glucose strands

Cotton fibres represent the purest natural form of cellulose, containing more than 90% of this polysaccharide.
Compared to starch, cellulose is also much more crystalline. Whereas starch undergoes a crystalline to amorphous transition when heated beyond 60–70 °C in water (as in cooking), cellulose requires a temperature of 320 °C and pressure of 25 MPa to become amorphous in water.[16]
Several different crystalline structures of cellulose are known, corresponding to the location of hydrogen bonds between and within strands. Natural cellulose is cellulose I, with structures Iα and Iβ. Cellulose produced by bacteria and algae is enriched in Iα while cellulose of higher plants consists mainly of Iβ. Cellulose in regenerated cellulose fibers is cellulose II. The conversion of cellulose I to cellulose II is irreversible, suggesting that cellulose I is metastable and cellulose II is stable. With various chemical treatments it is possible to produce the structures cellulose III and cellulose IV.[17]
Many properties of cellulose depend on its chain length or degree of polymerization, the number of glucose units that make up one polymer molecule. Cellulose from wood pulp has typical chain lengths between 300 and 1700 units; cotton and other plant fibers as well as bacterial cellulose have chain lengths ranging from 800 to 10,000 units.[6] Molecules with very small chain length resulting from the breakdown of cellulose are known as cellodextrins; in contrast to long-chain cellulose, cellodextrins are typically soluble in water and organic solvents.
Cellulose contains 44.44% carbon, 6.17% hydrogen, and 49.39% oxygen. The chemical formula of cellulose is (C6H10O5)n where n is the degree of polymerization and represents the number of glucose groups.[18]
Plant-derived cellulose is usually found in a mixture with hemicellulose, lignin, pectin and other substances, while bacterial cellulose is quite pure, has a much higher water content and higher tensile strength due to higher chain lengths.[6]:3384
Cellulose is soluble in Schweizer's reagent, cupriethylenediamine (CED), cadmiumethylenediamine (Cadoxen), N-methylmorpholine N-oxide, and lithium chloride / dimethylacetamide.[19] This is used in the production of regenerated celluloses (such as viscose and cellophane) from dissolving pulp. Cellulose is also soluble in many kinds of ionic liquids.[20]
Cellulose consists of crystalline and amorphous regions. By treating it with strong acid, the amorphous regions can be broken up, thereby producing nanocrystalline cellulose, a novel material with many desirable properties.[21] Recently, nanocrystalline cellulose was used as the filler phase in bio-based polymer matrices to produce nanocomposites with superior thermal and mechanical properties.[22]
Processing
Assay
Given a cellulose-containing material, the carbohydrate portion that does not dissolve in a 17.5% solution of sodium hydroxide at 20 °C is α cellulose, which is true cellulose.[clarification needed] Acidification of the extract precipitates β cellulose. The portion that dissolves in base but does not precipitate with acid is γ cellulose.[citation needed]
Cellulose can be assayed using a method described by Updegraff in 1969, where the fiber is dissolved in acetic and nitric acid to remove lignin, hemicellulose, and xylosans. The resulting cellulose is allowed to react with anthrone in sulfuric acid. The resulting coloured compound is assayed spectrophotometrically at a wavelength of approximately 635 nm.
In addition, cellulose is represented by the difference between acid detergent fiber (ADF) and acid detergent lignin (ADL).
Luminescent conjugated oligothiophenes can also be used to detect cellulose using fluorescence microscopy or spectrofluorometric methods.[23]
Biosynthesis
In vascular plants cellulose is synthesized at the plasma membrane by rosette terminal complexes (RTCs). The RTCs are hexameric protein structures, approximately 25 nm in diameter, that contain the cellulose synthase enzymes that synthesise the individual cellulose chains.[24] Each RTC floats in the cell's plasma membrane and "spins" a microfibril into the cell wall.
RTCs contain at least three different cellulose synthases, encoded by CesA genes, in an unknown stoichiometry.[25] Separate sets of CesA genes are involved in primary and secondary cell wall biosynthesis. There are known to be about seven subfamilies in the CesA superfamily. These cellulose synthases use UDP-glucose to form the β(1→4)-linked cellulose.[26]
Cellulose synthesis requires chain initiation and elongation, and the two processes are separate.
CesA glucosyltransferase initiates cellulose polymerization using a steroid primer, sitosterol-beta-glucoside, and UDP-glucose.[27]Cellulose synthase utilizes UDP-D-glucose precursors to elongate the growing cellulose chain. A cellulase may function to cleave the primer from the mature chain.
Cellulose is also synthesised by tunicate animals, particularly in the tests of ascidians (where the cellulose was historically termed "tunicine" (tunicin)).[28]
Breakdown (cellulolysis)
Cellulolysis is the process of breaking down cellulose into smaller polysaccharides called cellodextrins or completely into glucose units; this is a hydrolysis reaction. Because cellulose molecules bind strongly to each other, cellulolysis is relatively difficult compared to the breakdown of other polysaccharides.[29] However, this process can be significantly intensified in a proper solvent, e.g. in an ionic liquid.[30]
Most mammals have limited ability to digest dietary fiber such as cellulose. Some ruminants like cows and sheep contain certain symbiotic anaerobic bacteria (like Cellulomonas) in the flora of the rumen, and these bacteria produce enzymes called cellulases that help the microorganism to digest cellulose; the breakdown products are then used by the bacteria for proliferation. The bacterial mass is later digested by the ruminant in its digestive system (stomach and small intestine). Horses use cellulose in their diet by fermentation in their hindgut via symbiotic bacteria which produce cellulase to digest cellulose.[citation needed] Similarly, some termites contain in their hindguts certain flagellate protozoa producing such enzymes, whereas others contain bacteria or may produce cellulase.[31]
The enzymes used to cleave the glycosidic linkage in cellulose are glycoside hydrolases including endo-acting cellulases and exo-acting glucosidases. Such enzymes are usually secreted as part of multienzyme complexes that may include dockerins and carbohydrate-binding modules.[32]
Breakdown (thermolysis)
At temperatures above 350 °C, cellulose undergoes thermolysis (also called ‘pyrolysis’), decomposing into solid char, vapors, aerosols, and gases such as carbon dioxide.[33] Maximum yield of vapors which condense to a liquid called bio-oil is obtained at 500 °C.[34]
Semi-crystalline cellulose polymers react at pyrolysis temperatures (350–600 °C) in a few seconds; this transformation has been shown to occur via a solid-to-liquid-to-vapor transition, with the liquid (called intermediate liquid cellulose or molten cellulose) existing for only a fraction of a second.[35] Glycosidic bond cleavage produces short cellulose chains of two-to-seven monomers comprising the melt. Vapor bubbling of intermediate liquid cellulose produces aerosols, which consist of short chain anhydro-oligomers derived from the melt.[36]
Continuing decomposition of molten cellulose produces volatile compounds including levoglucosan, furans, pyrans, light oxygenates and gases via primary reactions.[37] Within thick cellulose samples, volatile compounds such as levoglucosan undergo ‘secondary reactions’ to volatile products including pyrans and light oxygenates such as glycolaldehyde.[38]
Hemicellulose
Hemicellulose is a polysaccharide related to cellulose that comprises about 20% of the biomass of most plants. In contrast to cellulose, hemicellulose is derived from several sugars in addition to glucose, especially xylose but also including mannose, galactose, rhamnose, and arabinose. Hemicellulose consists of shorter chains – between 500 and 3000 sugar units.[39] Furthermore, hemicellulose is branched, whereas cellulose is unbranched.
Derivatives
The hydroxyl groups (-OH) of cellulose can be partially or fully reacted with various reagents to afford derivatives with useful properties like mainly cellulose esters and cellulose ethers (-OR). In principle, though not always in current industrial practice, cellulosic polymers are renewable resources.
Ester derivatives include:
| Cellulose ester | Reagent | Example | Reagent | Group R |
|---|---|---|---|---|
| Organic esters | Organic acids | Cellulose acetate | Acetic acid and acetic anhydride | H or -(C=O)CH3 |
| Cellulose triacetate | Acetic acid and acetic anhydride | -(C=O)CH3 | ||
| Cellulose propionate | Propionic acid | H or -(C=O)CH2CH3 | ||
| Cellulose acetate propionate (CAP) | Acetic acid and propanoic acid | H or -(C=O)CH3 or -(C=O)CH2CH3 | ||
| Cellulose acetate butyrate (CAB) | Acetic acid and butyric acid | H or -(C=O)CH3 or -(C=O)CH2CH2CH3 | ||
| Inorganic esters | Inorganic acids | Nitrocellulose (cellulose nitrate) | Nitric acid or another powerful nitrating agent | H or -NO2 |
| Cellulose sulfate | Sulfuric acid or another powerful sulfuring agent | H or -SO3H |
The cellulose acetate and cellulose triacetate are film- and fiber-forming materials that find a variety of uses. The nitrocellulose was initially used as an explosive and was an early film forming material. With camphor, nitrocellulose gives celluloid.
Ether derivatives include:
| Cellulose ethers | Reagent | Example | Reagent | Group R = H or | Water solubility | Application | E number |
|---|---|---|---|---|---|---|---|
| Alkyl | Halogenoalkanes | Methylcellulose | Chloromethane | -CH3 | Cold water-soluble | E461 | |
| Ethylcellulose | Chloroethane | -CH2CH3 | Water-insoluble | A commercial thermoplastic used in coatings, inks, binders, and controlled-release drug tablets | E462 | ||
| Ethyl methyl cellulose | Chloromethane and chloroethane | -CH3 or -CH2CH3 | E465 | ||||
| Hydroxyalkyl | Epoxides | Hydroxyethyl cellulose | Ethylene oxide | -CH2CH2OH | Cold/hot water-soluble | Gelling and thickening agent | |
Hydroxypropyl cellulose (HPC) | Propylene oxide | -CH2CH(OH)CH3 | Cold water-soluble | E463 | |||
| Hydroxyethyl methyl cellulose | Chloromethane and ethylene oxide | -CH3 or -CH2CH2OH | Cold water-soluble | Production of cellulose films | |||
Hydroxypropyl methyl cellulose (HPMC) | Chloromethane and propylene oxide | -CH3 or -CH2CH(OH)CH3 | Cold water-soluble | Viscosity modifier, gelling, foaming and binding agent | E464 | ||
| Ethyl hydroxyethyl cellulose | Chloroethane and ethylene oxide | -CH2CH3 or—CH2CH2OH | E467 | ||||
| Carboxyalkyl | Halogenated carboxylic acids | Carboxymethyl cellulose (CMC) | Chloroacetic acid | -CH2COOH | Cold/Hot water-soluble | Often used as its sodium salt, sodium carboxymethyl cellulose (NaCMC) | E466 |
The sodium carboxymethyl cellulose can be cross-linked to give the croscarmellose sodium (E468) for use as a disintegrant in pharmaceutical formulations.
Applications
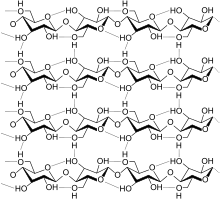
A strand of cellulose (conformation Iα), showing the hydrogen bonds (dashed) within and between cellulose molecules.
Cellulose for industrial use is mainly obtained from wood pulp and cotton.[6] The kraft process is used to separate cellulose from lignin, another major component of plant matter.
- Paper products: Cellulose is the major constituent of paper, paperboard, and card stock.
- Fibers: Cellulose is the main ingredient of textiles made from cotton, linen, and other plant fibers. It can be turned into rayon, an important fiber that has been used for textiles since the beginning of the 20th century. Both cellophane and rayon are known as "regenerated cellulose fibers"; they are identical to cellulose in chemical structure and are usually made from dissolving pulp via viscose. A more recent and environmentally friendly method to produce a form of rayon is the Lyocell process.
- Consumables: Microcrystalline cellulose (E460i) and powdered cellulose (E460ii) are used as inactive fillers in drug tablets[40] and a wide range of soluble cellulose derivatives, E numbers E461 to E469, are used as emulsifiers, thickeners and stabilizers in processed foods. Cellulose powder is, for example, used in Parmesan cheese to prevent caking inside the package. Cellulose occurs naturally in some foods and is an additive in manufactured foods, contributing an indigestible component used for texture and bulk, potentially aiding in defecation.[41]
- Science: Cellulose is used in the laboratory as a stationary phase for thin layer chromatography. Cellulose fibers are also used in liquid filtration, sometimes in combination with diatomaceous earth or other filtration media, to create a filter bed of inert material.
- Energy crops: The major combustible component of non-food energy crops is cellulose, with lignin second. Non-food energy crops produce more usable energy than edible energy crops (which have a large starch component), but still compete with food crops for agricultural land and water resources.[42] Typical non-food energy crops include industrial hemp (though outlawed in some countries), switchgrass, Miscanthus, Salix (willow), and Populus (poplar) species.
- Biofuel: TU-103, a strain of Clostridium bacteria found in zebra waste, can convert nearly any form of cellulose into butanol fuel.[43][44]
- Building material: Hydroxyl bonding of cellulose in water produces a sprayable, moldable material as an alternative to the use of plastics and resins. The recyclable material can be made water- and fire-resistant. It provides sufficient strength for use as a building material.[45]Cellulose insulation made from recycled paper is becoming popular as an environmentally preferable material for building insulation. It can be treated with boric acid as a fire retardant.
- Miscellaneous: Cellulose can be converted into cellophane, a thin transparent film. It is the base material for the celluloid that was used for photographic and movie films until the mid-1930s. Cellulose is used to make water-soluble adhesives and binders such as methyl cellulose and carboxymethyl cellulose which are used in wallpaper paste. Cellulose is further used to make hydrophilic and highly absorbent sponges. Cellulose is the raw material in the manufacture of nitrocellulose (cellulose nitrate) which is used in smokeless gunpowder.
- Pharmaceuticals: Cellulose derivatives, such as microcrystalline cellulose (MCC), have the advantages of retaining water, being a stabilizer and thickening agent, and in reinforcement of drug tablets.[46]
See also
- Microbial cellulose
- Zeoform
References
^ Nishiyama, Yoshiharu; Langan, Paul; Chanzy, Henri (2002). "Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction". J. Am. Chem. Soc. 124 (31): 9074–82. doi:10.1021/ja0257319. PMID 12149011..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcd "NIOSH Pocket Guide to Chemical Hazards #0110". National Institute for Occupational Safety and Health (NIOSH).
^ ab Crawford, R. L. (1981). Lignin biodegradation and transformation. New York: John Wiley and Sons. ISBN 0-471-05743-6.
^ Updegraff DM (1969). "Semimicro determination of cellulose in biological materials". Analytical Biochemistry. 32 (3): 420–424. doi:10.1016/S0003-2697(69)80009-6. PMID 5361396.
^ Romeo, Tony (2008). Bacterial biofilms. Berlin: Springer. pp. 258–263. ISBN 978-3-540-75418-3.
^ abcde Klemm, Dieter; Heublein, Brigitte; Fink, Hans-Peter; Bohn, Andreas (2005). "Cellulose: Fascinating Biopolymer and Sustainable Raw Material". Angew. Chem. Int. Ed. 44 (22): 3358–93. doi:10.1002/anie.200460587. PMID 15861454.
^ Cellulose. (2008). In Encyclopædia Britannica. Retrieved January 11, 2008, from Encyclopædia Britannica Online.
^ Chemical Composition of Wood. ipst.gatech.edu.
^ Piotrowski, Stephan and Carus, Michael (May 2011) Multi-criteria evaluation of lignocellulosic niche crops for use in biorefinery processes. nova-Institut GmbH, Hürth, Germany.
^ Payen, A. (1838) "Mémoire sur la composition du tissu propre des plantes et du ligneux" (Memoir on the composition of the tissue of plants and of woody [material]), Comptes rendus, vol. 7, pp. 1052–1056. Payen added appendices to this paper on December 24, 1838 (see: Comptes rendus, vol. 8, p. 169 (1839)) and on February 4, 1839 (see: Comptes rendus, vol. 9, p. 149 (1839)). A committee of the French Academy of Sciences reviewed Payen's findings in : Jean-Baptiste Dumas (1839) "Rapport sur un mémoire de M. Payen, relatif à la composition de la matière ligneuse" (Report on a memoir of Mr. Payen, regarding the composition of woody matter), Comptes rendus, vol. 8, pp. 51–53. In this report, the word "cellulose" is coined and author points out the similarity between the empirical formula of cellulose and that of "dextrine" (starch). The above articles are reprinted in: Brongniart and Guillemin, eds., Annales des sciences naturelles ..., 2nd series, vol. 11 (Paris, France: Crochard et Cie., 1839), pp. 21–31.
^ Young, Raymond (1986). Cellulose structure modification and hydrolysis. New York: Wiley. ISBN 0-471-82761-4.
^ Kobayashi, Shiro; Kashiwa, Keita; Shimada, Junji; Kawasaki, Tatsuya; Shoda, Shin-ichiro (1992). "Enzymatic polymerization: The first in vitro synthesis of cellulose via nonbiosynthetic path catalyzed by cellulase". Makromolekulare Chemie. Macromolecular Symposia. 54–55 (1): 509–518. doi:10.1002/masy.19920540138.
^ Bishop, Charles A., ed. (2007). Vacuum deposition onto webs, films, and foils. p. 165. ISBN 0-8155-1535-9.
^ Dauenhauer, Paul; Krumm, Christoph; Pfaendtner, Jim (2016). "Millisecond Pulsed Films Unify the Mechanisms of Cellulose Fragmentation". Chemistry of Materials. 28 (1): 0001. doi:10.1021/acs.chemmater.6b00580.
^ Wymer, Charles E. (1994). "Ethanol from lignocellulosic biomass: Technology, economics, and opportunities". Bioresource Technology. 50 (1): 5. doi:10.1016/0960-8524(94)90214-3. Retrieved 28 October 2016.
^ Deguchi, Shigeru; Tsujii, Kaoru; Horikoshi, Koki (2006). "Cooking cellulose in hot and compressed water". Chemical Communications (31): 3293. doi:10.1039/b605812d.
^ Structure and morphology of cellulose Archived April 26, 2009, at the Wayback Machine. by Serge Pérez and William Mackie, CERMAV-CNRS, 2001. Chapter IV.
^ Chapter 2: Chemical Composition and Structure of Natural Lignocellulose
^ Stenius, Per (2000). "Ch. 1". Forest Products Chemistry. Papermaking Science and Technology. Vol. 3. Finland: Fapet OY. p. 35. ISBN 952-5216-03-9.
^ Wang, Hui; Gurau, Gabriela; Rogers, Robin D. (2012). "Ionic liquid processing of cellulose". Chemical Society Reviews. 41 (4): 1519–37. doi:10.1039/C2CS15311D. PMID 22266483.
^ Peng, B. L., Dhar, N., Liu, H. L. and Tam, K. C. (2011). "Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective" (PDF). The Canadian Journal of Chemical Engineering. 89 (5): 1191–1206. doi:10.1002/cjce.20554.CS1 maint: Multiple names: authors list (link)
^ Pranger, L.; Tannenbaum, R. (2008). "Biobased Nanocomposites Prepared by in Situ Polymerization of Furfuryl Alcohol with Cellulose Whiskers or Montmorillonite Clay". Macromolecules. 41 (22): 8682–8687. doi:10.1021/ma8020213.
^ Choong, Ferdinand X.; Bäck, Marcus; Steiner, Svava E.; Melican, Keira; Nilsson, K. Peter R.; Edlund, Ulrica; Richter-Dahlfors, Agneta (19 October 2016). "Nondestructive, real-time determination and visualization of cellulose, hemicellulose and lignin by luminescent oligothiophenes". Scientific Reports. 6: 35578. doi:10.1038/srep35578. PMC 5069672. PMID 27759105. Retrieved 19 October 2016.
^ Kimura, S; Laosinchai, W; Itoh, T; Cui, X; Linder, CR; Brown Jr, RM (1999). "Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant vigna angularis". The Plant Cell. 11 (11): 2075–86. doi:10.2307/3871010. JSTOR 3871010. PMC 144118. PMID 10559435.
^ Taylor, N. G. (2003). "Interactions among three distinct CesA proteins essential for cellulose synthesis". Proceedings of the National Academy of Sciences. 100 (3): 1450–1455. doi:10.1073/pnas.0337628100. PMC 298793. PMID 12538856.
^ Richmond, Todd A; Somerville, Chris R (October 2000). "The Cellulose Synthase Superfamily". Plant Physiology. 124 (2): 495–498. doi:10.1104/pp.124.2.495. PMC 1539280. PMID 11027699. Retrieved 14 December 2014.
^ Peng, L; Kawagoe, Y; Hogan, P; Delmer, D (2002). "Sitosterol-beta-glucoside as primer for cellulose synthesis in plants". Science. 295 (5552): 147–50. doi:10.1126/science.1064281. PMID 11778054.
^ Endean, R (1961). "The Test of the Ascidian, Phallusia mammillata" (PDF). Quarterly Journal of Microscopical Science. 102 (1): 107–117.
^ Barkalow, David G.; Whistler, Roy L. (2014). "Cellulose". AccessScience. doi:10.1036/1097-8542.118200.
^ Ignatyev, Igor; Doorslaer, Charlie Van; Mertens, Pascal G.N.; Binnemans, Koen; Vos, Dirk. E. de (2011). "Synthesis of glucose esters from cellulose in ionic liquids". Holzforschung. 66 (4): 417–425. doi:10.1515/hf.2011.161.
^ Tokuda, G; Watanabe, H (22 June 2007). "Hidden cellulases in termites: revision of an old hypothesis". Biology Letters. 3 (3): 336–339. doi:10.1098/rsbl.2007.0073. PMC 2464699. PMID 17374589.
^ Brás, Natércia (2008). "Carbohydrate Binding Modules from family 11: Understanding the binding mode of polysaccharides". International Journal of Quantum Chemistry. 108 (11): 2030–2040. doi:10.1002/qua.21755.
^ Mettler, Matthew S.; Vlachos, Dionisios G.; Dauenhauer, Paul J. (2012). "Top Ten Fundamental Challenges of Biomass Pyrolysis for Biofuels". Energy & Environmental Science. 5 (7): 7797. doi:10.1039/C2EE21679E.
^ Czernik, S.; Bridgwater, A. V. (2004). "Overview of Applications of Biomass Fast Pyrolysis Oil". Energy & Fuels. Energy & Fuels, American Chemical Society. 18 (2): 590–598. doi:10.1021/ef034067u.
^ Dauenhauer, Paul J.; Colby, Joshua L.; Balonek, Christine M.; Suszynski, Wieslaw J.; Schmidt, Lanny D. (2009). "Reactive Boiling of Cellulose for Integrated Catalysis through an Intermediate Liquid". Green Chemistry. 11 (10): 1555. doi:10.1039/B915068B.
^ Teixeira, Andrew R.; Mooney, Kyle G.; Kruger, Jacob S.; Williams, C. Luke; Suszynski, Wieslaw J.; Schmidt, Lanny D.; Schmidt, David P.; Dauenhauer, Paul J. (2011). "Aerosol Generation by Reactive Boiling Ejection of Molten Cellulose". Energy & Environmental Science. Energy & Environmental Science, Royal Society of Chemistry. 4 (10): 4306. doi:10.1039/C1EE01876K.
^ Mettler, Matthew S.; Mushrif, Samir H.; Paulsen, Alex D.; Javadekar, Ashay D.; Vlachos, Dionisios G.; Dauenhauer, Paul J. (2012). "Revealing pyrolysis chemistry for biofuels production: Conversion of cellulose to furans and small oxygenates". Energy Environ. Sci. 5: 5414–5424. doi:10.1039/C1EE02743C.
^ Mettler, Matthew S.; Paulsen, Alex D.; Vlachos, Dionisios G.; Dauenhauer, Paul J. (2012). "Pyrolytic Conversion of Cellulose to Fuels: Levoglucosan Deoxygenation via Elimination and Cyclization within Molten Biomass". Energy & Environmental Science. 5 (7): 7864. doi:10.1039/C2EE21305B.
^ Gibson LJ (2013). "The hierarchical structure and mechanics of plant materials". Journal of the Royal Society Interface. 9 (76): 2749–2766. doi:10.1098/rsif.2012.0341. PMC 3479918. PMID 22874093.
^
Weiner, Myra L.; Kotkoskie, Lois A. (2000). Excipient Toxicity and Safety. New York: Dekker. p. 210. ISBN 978-0-8247-8210-8.
^ Dhingra, D; Michael, M; Rajput, H; Patil, R. T. (2011). "Dietary fibre in foods: A review". Journal of Food Science and Technology. 49 (3): 255–266. doi:10.1007/s13197-011-0365-5. PMC 3614039. PMID 23729846.
^ Holt-Gimenez, Eric (2007). Biofuels: Myths of the Agrofuels Transition. Backgrounder. Institute for Food and Development Policy, Oakland, CA. 13:2 "Archived copy". Archived from the original on 2013-09-05. Retrieved 2013-09-05.CS1 maint: Archived copy as title (link)
"Archived copy". Archived from the original on 2013-09-06. Retrieved 2013-09-05.CS1 maint: Archived copy as title (link)
^ Hobgood Ray, Kathryn (August 25, 2011). "Cars Could Run on Recycled Newspaper, Tulane Scientists Say". Tulane University news webpage. Tulane University. Retrieved March 14, 2012.
^ Balbo, Laurie (January 29, 2012). "Put a Zebra in Your Tank: A Chemical Crapshoot?". Greenprophet.com. Retrieved November 17, 2012.
^ "Zeoform: The eco-friendly building material of the future?". Gizmag.com. Retrieved 2013-08-30.
^ Thoorens, G; Krier, F; Leclercq, B; Carlin, B; Evrard, B (2014). "Microcrystalline cellulose, a direct compression binder in a quality by design environment--a review". International Journal of Pharmaceutics. 473 (1–2): 64–72. doi:10.1016/j.ijpharm.2014.06.055. PMID 24993785.
External links
 "Cellulose". Encyclopædia Britannica. 5 (11th ed.). 1911.
"Cellulose". Encyclopædia Britannica. 5 (11th ed.). 1911.
Structure and morphology of cellulose by Serge Pérez and William Mackie, CERMAV-CNRS
Cellulose, by Martin Chaplin, London South Bank University
Clear description of a cellulose assay method at the Cotton Fiber Biosciences unit of the USDA.
Cellulose films could provide flapping wings and cheap artificial muscles for robots – TechnologyReview.com- CDC - NIOSH Pocket Guide to Chemical Hazards - Cellulose